Per- and polyfluoroalkyl substances (PFAS) are a class of widely used chemicals that are pervasive and persistent in the environment. PFAS can be broadly classified as polymers and non-polymers. Most environmental investigations focus on non-polymer PFAS, which are primarily manufactured through two processes, electrochemical fluorination (ECF) and telomerization.
Differences between and within the ECF and telomerization processes lend distinguishing characteristics to the resulting PFAS found in the environment. Prior to 2000, longer carbon chain PFAS (e.g., PFCAs with seven or more fluorinated carbons, PFSAs with six or more fluorinated carbons) dominated the industry, with a transition from 2000 to 2015 to shorter chain and alternate structures. Many PFAS compounds released to the environment have complex structures that can degrade over time to stable sulfonate (e.g., PFOS) and carboxylate forms (e.g., PFOA).
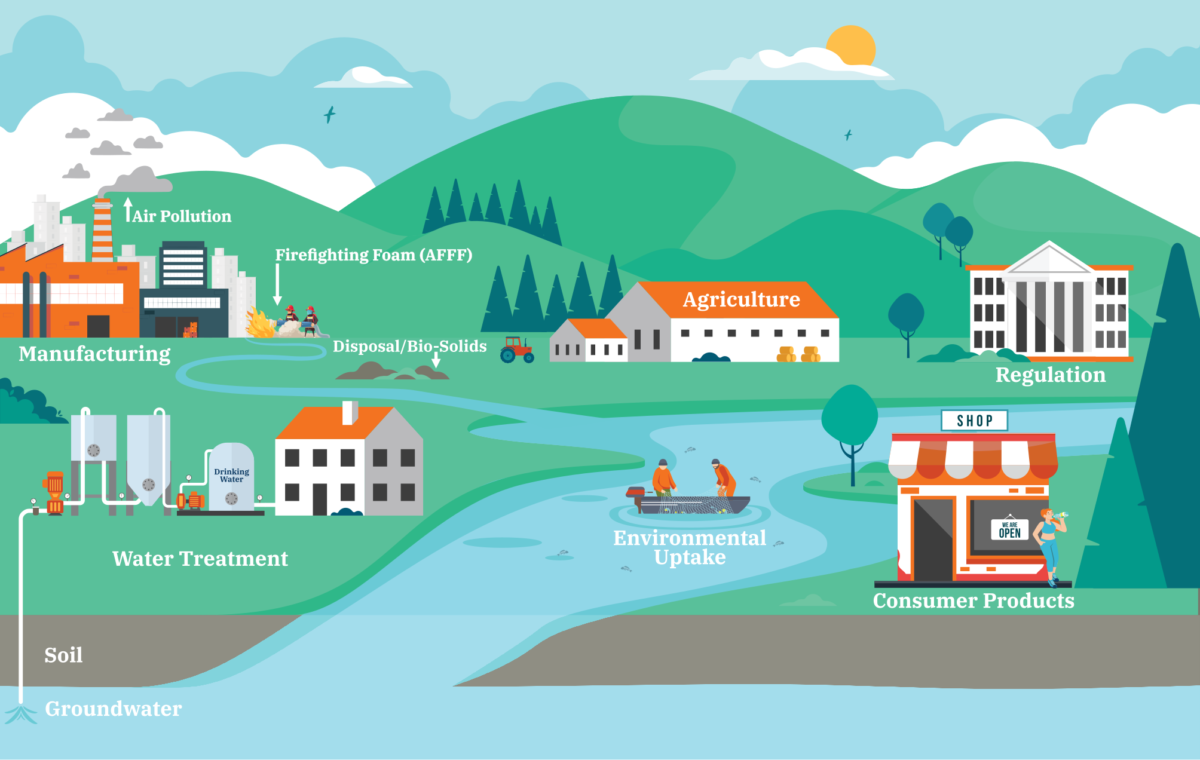
PFAS may be released to and travel through the environment in a variety of ways. The graphic below illustrates some of the ways that people may come into contact with PFAS. Click on each red dot to learn more about each pathway.